Enter your answers as whole numbers not words 1. The central atom is usually written first in the formula and is the least electronegative.
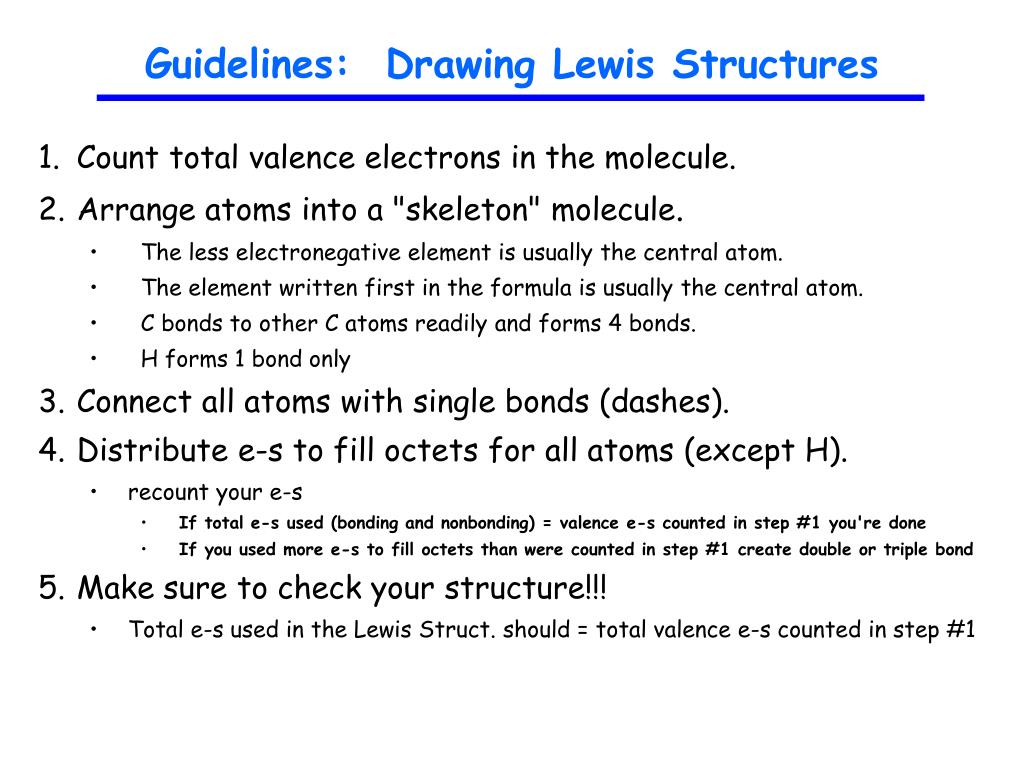
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949
Here we will be using the determined total number of valence electrons per atom and drawing them in the proper places.

. Determine the total number of valence electrons in the molecule. Count the total number of regions of electron density bonding and lone electron pairs around the central atom. For a polyatomic anion electrons are added to take into account the negative charge.
Sometimes the number of valence e- is odd. Add up the valence electrons of the atoms. WRITING LEWIS DOT STRUCTURES Lewis structure or formula shows electrondot symbols for the atoms the bonding pairs as lines and the lone pairs that fill each atoms outer level valence shell as pairs of dots.
Determine the total number of valence electrons. General rules for writing LEWIS structures for molecules. See the following Lewis dot structure diagrams for a few covalent compounds.
Its not perfect but it is a useful tool for scientists trying to understand how molecules will react and their. Review the rules for drawing Lewis dot structures and VSEPR theory and complete the pre-lab exercises before coming to lab. Write the skeletal structure.
-Add one electron for each negative charge. Count the number of valence electrons. Odd number of electrons.
Unpaired electrons are observed in odd electron molecules such as NO and NO2. If ionic treat each ion separately. The general rules for writing Lewis structures include the following.
Hydrogen 2 valence Boron 6 valence Aluminum 6 valence Gallium 6 valence Indium 6 valence Sometimes Exceptions. For a molecule simply sum up the valence electrons of the atoms present. How many lone pairs of electrons are around the central atom.
Draw the initial skeletal structure with atoms connected by single bonds. The specific contents of your model kit may vary but a typical kit. How many valence electrons were used to make the drawing.
Complete pairing of these e- is impossible and an octet around each atom cannot be achieved resonance structures 2. If covalent treat the entire molecule. Add one for each - charge extra electron.
Lewis Structures Rules for Drawing Lewis Structure. For the most part Lewis structures reflect what is found when we gather experimental data for molecules in the laboratory. For a molecule uncharged that count is the correct.
Count the total number of valence electrons in the structure Remember Group of Valence electrons 2. Copyright 2001 L. It should be the most symmetric structure you can draw.
Generally electrons are paired. -Atoms that occur once or first are usually central. The central atom most of the time is the more positive atom.
Examples for Drawing Lewis Dot Structure for Covalent Bonds. -Hydrogen cannot be central. Rules for drawing Lewis structures.
Occurs when there are fewer than eight valence electrons around an atom resonance structures 3. The following rules are given to assist you. Lewis structures can be drawn with a set of general rules that give us a pretty good idea of how valence electrons are distributed around a molecule.
Using the rules learned in class draw the Lewis structure of SeO2 and use it to answer the following questions. Nitrogen 5 valence Phosphorus 10 valence. Make sure that H has only 1 bond and that the other atoms do not exceed their normal number 2 bonds to O 3 bonds to N 4 bonds to C and that no atom in the molecule has more than 4 bonds.
Two electrons are shared between two atoms constitutes a single bond. Consult the molecular formula and sum up all the valence electrons from the separate atoms. Ø Double and triple bonds count as ONE REGION OF HIGH ELECTRON DENSITY.
Ø An unpaired electron counts as ONE REGION OF HIGH ELECTRON DENSITY. Determine whether the compound is covalent or ionic. -Subtract one electron for each positive charge.
Once the electron dot structure is complete translate this structure into a Lewis structure. First of all a correct count of all valence electrons is essential. The least electronegative atom will be central.
General Rules for Drawing Covalently Bonded Lewis Structures 1. LEWIS STRUCTURES General Rules for Drawing Lewis Structures 1. The video covers the basic Lewis structures youll see in an introductory chemistry class.
And recall that for main group elements the valence electrons can be determined from the group number. Rules for Drawing Lewis Structures First sum the number of valence electrons from each atom Guess at the skeletal structure. Less than an octet of valence electrons.
Determine the total number of valence electrons in the molecule or ion. Compounds of low electronegativity metals with high electronegativity nonmetals ΔEN 16 are ionic as are compounds of metals with polyatomic anions. Determine the central atom and write the skeleton structure of the molecule.
Shared pairs of electrons are drawn as lines between atoms while lone pairs of electrons are drawn as dots next to atoms. When constructing a Lewis diagram keep in mind the octet rule which refers to the tendency of atoms to gain lose or share electrons until they are surrounded by eight valence electrons an octet. The model kits use color-coded atom centers with pegs to represent electron pairs arranged in the more common geometries.
If the charge is negative add electrons and if the charge is positive subtract electrons Step 2. Decide on a skeletal structure What is bonded to what The central atom is generally written first in the formula. Reference the How to Draw a Lewis Dot Structure for a Step by Step guide.
To do this you will need the periodic table. All valence electrons of the atoms in Lewis structures must be shown. All atoms want to fulfill the octet rule.
Subtract one for each charge missing electron. Four electrons shared is a double bond and six electrons shared is a triple bond. Given a chemical formula corresponding to a molecule or molecular ion draw a Lewis structure.
Draw the Lewis structure for the molecule or ion. Never Hyrdrogen Place a pair of electrons between all atoms Complete the octets of atoms on the outside. Draw a skeleton structure for the molecule or ion by joining atoms by a single bond to the central atom.
MUST FOLLOW IN ORDER. A video tutorial for how to draw Lewis Structures in five steps. One way to do this is to write the Lewis symbols for all of the atoms in the formula and count up all the dots.
Rules for Drawing Lewis Structures For Molecular Compounds 1 Calculate the total number of electrons for the structure by summing the valence electrons of each atom in the molecule. CBr 4-The least electronegative atom is central. The Group number in the Periodic Table the number of valence electrons for an atom.
Rules for Drawing Lewis Structures For molecules known to exist follow these rules to determine the Lewis structure. Lewis electron dot Structures.

Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421

I Can 2 I Can Draw A Lewis Structure Rules For Lewis Structures 1 Total Number Of Valance Electrons 2 Central Atom Always C Never H Rarely O Or Ppt Download

Lewis Structure Organic Chemistry Video Clutch Prep
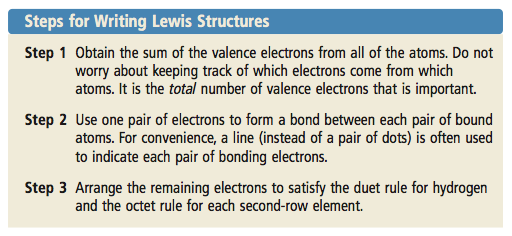


0 comments
Post a Comment